Avik Roy argues, at length, that regulations have stifled drug innovation. He wants to reform the drug-approval process:
[T]he system is oriented toward acute diseases, like contagious infections, in which symptoms appear rapidly and the effect of medication is also relatively quick. Such diseases were the most prevalent menace to public health when the federal government began regulating drugs in 1906. Today, however, the greatest dangers to long-term public health are chronic non-communicable diseases such as heart ailments, diabetes, stroke, and cancer. These conditions can persist for decades. That makes it more difficult to measure the true effects of a medication in the time scale of even the most wide-ranging of clinical trials.
He suggests gradations of approval:
A "conditional approval"’ approach would grant limited marketing authorization to new drugs after successful Phase II trials. Under conditional approval, patients most in need can benefit from a new drug, and companies can generate a modest amount of revenue that can help fund Phase III trials for full approval.
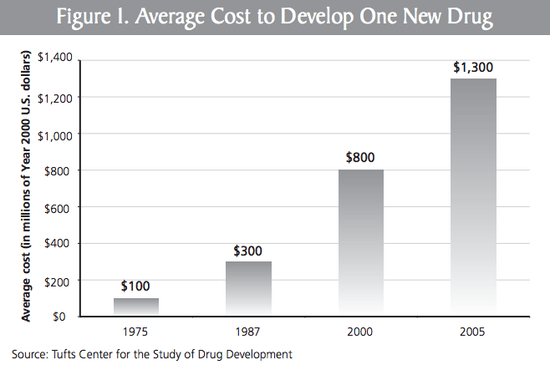
(Chart from Roy's new report (pdf) on drug costs)